Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 3 Electrochemistry. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Electrochemistry MCQs Pdf with Answers to know their preparation level. https://www.cbselabs.com/chemistry-mcqs-for-class-12-with-answers-chapter-3/
Electrochemistry Class 12 Chemistry MCQs Pdf
Electrochemistry Class 12 MCQ
1. The charge required for the reduction of 1 mol of MnO4– to MnO2 is
(a) 1 F
(b) 3 F
(c) 5 F
(d) 6 F
Answer
Answer: b
Electrochemistry MCQ Class 12
2. The cell reaction of the galvanic cell.
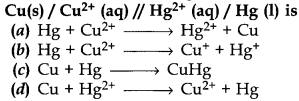
Answer
Answer: d
Electrochemistry MCQ Questions Class 12
3. Which of the following reaction is used to make fuel cell?
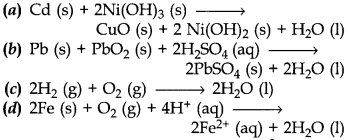
Answer
Answer: c
MCQ Of Electrochemistry Class 12
4. If limiting molar conductivity of Ca2+ and Cl– are 119.0 and 76.3 S cm2 mol-1, then the value of limiting molar conductivity of CaCl2 will be
(a) 195.3 S cm2 mol-1
(b) 271.6 S cm2 mol-1
(c) 43.3 S cm2 mol-1
(d) 314.3 S cm2 mol-1.
Answer
Answer: b
MCQ Questions For Class 12 Chemistry Chapter 3
5. NH4NC>3 is used in salt bridge because
(a) it forms a jelly like material with agar-agar.
(b) it is a weak electrolyte.
(c) it is a good conductor of electricity.
(d) the transport number of NH4+ and NO3– ions are almost equal.
Answer
Answer: d
Electrochemistry MCQ With Answers Pdf Download
6.
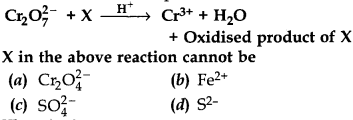
Answer
Answer: b
Class 12 Chemistry Chapter 3 MCQ
7. The reaction, 3ClO– (aq) → ClO3 (aq) + 2Cl– (aq) is an example of
(a) Oxidation reaction
(b) Reduction reaction
(c) Disproportionation reaction
(d) Decomposition reaction
Answer
Answer: c
Chemistry Class 12 Chapter 3 MCQ
8. The emf of the cell:
Ni / Ni2+ (1.0 M) // Au3+ (1.0 M) / Au (E° = -0.25 V for Ni2+/Ni; E° = 1.5 V for Au3+/Au) is
(a) 1.25 V
(b) -1.25 V
(c) 1.75 V
(d) 2.0 V
Answer
Answer: c
MCQ On Electrochemistry Class 12
9. The standard emf of a galvanic cell involving cell reaction with n = 2 is formed to be 0.295 V at 25° C. The equilibrium constant of the reaction would be
(a) 1.0 × 1010
(b) 2.0 × 1011
(c) 4.0 × 1012
(d) 1.0 × 102
[Given F = 96500 (mol-1); R = 8.314 JK-1 mol-1]
Answer
Answer: a
MCQ On Electrochemistry Class 12 With Answers
10. If E°Fe2+/Fe = -0.441 V and E°Fe2+/Fe2+ = 0.771 V, the standard EMF of the reaction,
Fe + 2Fe3+ → 3Fe2+ will be
(a) 1.212 V
(b) 0.111 V
(C) 0.330 V
(d) 1.653 V
Answer
Answer: a
We hope the given Chemistry MCQs for Class 12 with Answers Chapter 3 Electrochemistry will help you. If you have any query regarding CBSE Class 12 Chemistry Electrochemistry MCQs Pdf, drop a comment below and we will get back to you at the earliest.